
Since there is no lone pair present on the central atom in the CO2 lewis dot structure. But the whole molecule of CO2 is non-polar in nature.Īs we know, the molecular geometry of CO2 is linear and electron geometry is also linear. In the case of CO2, the C=O bond is polar in nature due to the difference in electronegativity between them. Therefore, oxygen has a higher tendency to attract an electron to itself than carbon.Īlso, the electronegativity difference between carbon and oxygen is more than 0.5, and according to the Pauling scale if the electronegativity difference between atoms is higher than 0.5 then the bond between that atoms behaves as polar.īut it doesn’t necessary if some bond is polar then the whole molecule can also be polar. Now, look at the electronegativity of carbon and oxygen.Īs carbon electronegativity is around 2.6 and for oxygen, it is around 3.45. If the electronegativity difference between the atoms is high then the polarity will also be higher. Electronegativity means the tendency of an atom to attract electrons towards itself.

Because the polarity is directly proportional to the difference of electronegativity created by atoms or molecules. It directly influences the polarity nature of any atom or molecule. Three factors that indicate the polarity of CO2 1. Since, the central Carbon (C) atom is surrounded by 2 regions of electron density, according to VSEPR theory, “the maximum distance two regions of electron density can get away from affords a geometry called Linear”.
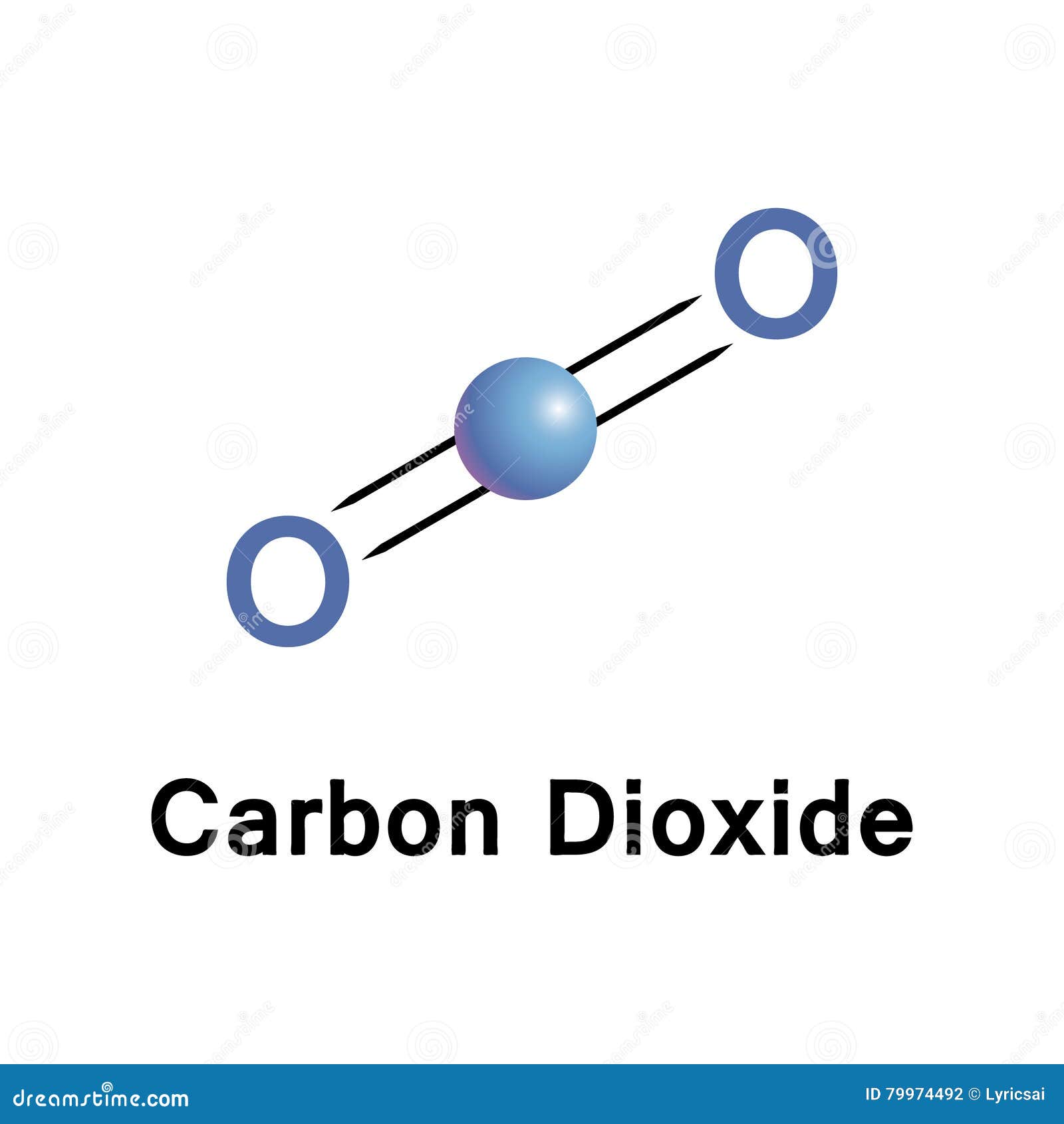
The electron geometry for CO2 is also linear. So, in a CO2 molecule, the carbon central atom has no lone pair, and it is attached with only bonding pairs, hence, for keeping the repulsive force least generated by the bonding pairs around the central atom, CO2 acquires the linear molecular geometry or shape. It doesn’t matter how many lone pair are on outer atoms, only thing is matter around the central atom. VSEPR theory only predict the geometry based on electron pair that are around the Central atom. Based on VSEPR theory, electrons pairs around the central carbon atom are going to repel each other, so, those two oxygen atoms are going to push far as much as they can to either side of the central atom and that makes, CO2 is a linear molecule.Īs you see in the above figure, the bond pair on both sides of the carbon central atom are repelling each other, because of this, both side oxygen atoms are pushed far away from each other in a straight line, therefore, the overall molecular geometry of CO2 will be linear.

The central atom carbon (C) is bonded with two oxygen (O) atoms via a double bond and it has zero lone pair.


 0 kommentar(er)
0 kommentar(er)
